Electric Charges and Fields
Electric charge is a property of subatomic particles that lets us know if they experience forces from electric or magnetic fields. Electric charges can either be positive or negative. Positive charges are associated with protons, subatomic particles in the nucleus of an atom, whereas negative charges are associated with electrons, which orbit the nucleus. The distinction in charges is what creates the force of attraction between the particles. A negative charge comes from something with an excess of electrons relative to protons, and a positive charge comes from something with an excess of protons relative to electrons.
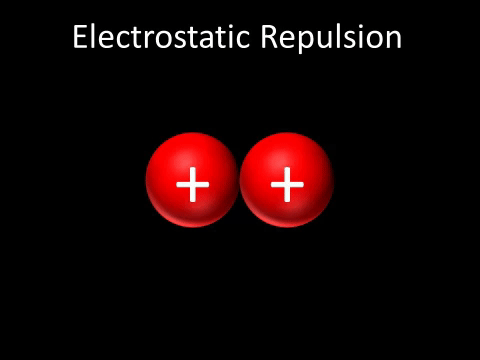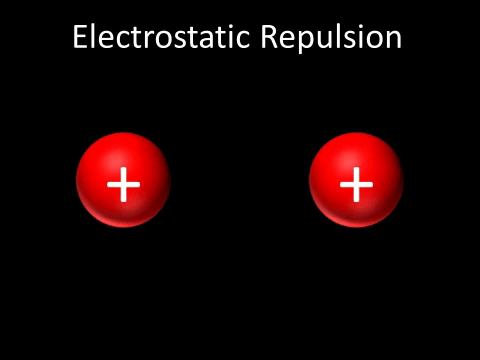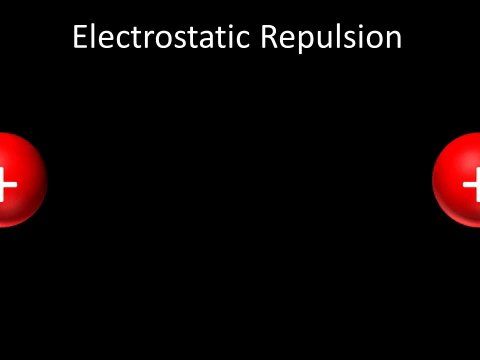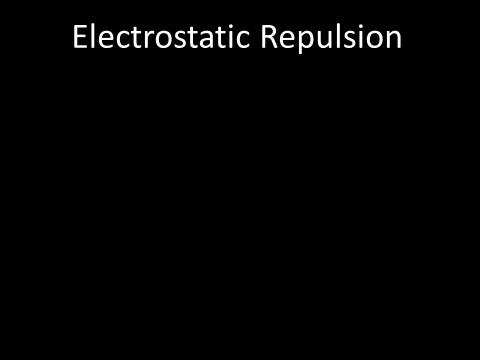
Coulomb's law of attraction states that if there are two opposite charges, they will attract each other by a force that decreases with distance. It also states that the same charges will repel each other with a force that also decreases with distance. In basic terms, opposites attract and likes repel.
A coulomb, C, is a measure of electric charge, created by this equation:
Q = I t
where Q is the electric charge, I is the electric current, and t is time. One coulomb is the quantity of charge measured per second.
Due to their incredibly small size, subatomic particles do not experience gravity or air resistance forces. These forces acting on them are so small in magnitude that they are to be considered negligible.
There are a few properties of charges that are important to understanding their behavior when interacting with each other.
Additivity of electric charges: when there are multiple charges near each other, the total electric charge can be measured with the sum of all their magnitudes. If you have a charge with a measure of +3 C, and another with a measure of -2 C, the total electric charge will be +1.
Conservation of electric charge: the total electric charge will remain unchanged in an isolated system. This also means that the total electric charge will remain the same over time.
Quantization of electric charges: electric charges come from a culmination of individual charges called elementary charges. The smallest elementary charge is that carried by one electron, which is -1.6 x 10⁻¹⁹ C.
The act of giving or removing a charge is called charging. There are three different ways to give something that is uncharged a charge: friction (triboelectric), conduction, and induction.
Friction charging happens when two things are rubbed together. One of the objects loses electrons and the other gains electrons, causing an electrostatic attraction. The electrons in the object that gained electrons are attracted to the protons in the other object, and the opposite electrons are repelled, creating an attraction. If you rub a balloon against your hair, the hair transfers electrons to the balloon, creating a difference in charge, which is why the balloon sticks to your hair.
Charging by conduction happens when one thing is negatively charged, and one is uncharged. When touching one another, the charged object will discharge electrons into the other object to achieve stability. Induction is also when one object is charged and one is not. When brought in proximity with one another, there is a redistribution of charge, as the electrons are repelled from the other object, causing an induced charge.
An electric field is a vector field that shows a charge’s behavior with the space around it. The direction of an electric field can be found with the total direction of all the field lines. The field lines are lines that show what direction the charge’s influence is in. Two charges with the same magnitude will have the same amount of field lines. The formula for the strength of an electric field is as follows:
E = F / Q
where E is the electric field strength, F is force, and Q is the charge. On an electric field, positive charges create field lines and negative charges attract them, creating a relationship between opposite charges on an electric field. When two of the same charges are in an electric field, the field lines do not touch, as the charges repel.
These fields are extremely useful for understanding the relationships between charges and the influences on the rest of the environment.