Lewis Structures
A Lewis structure is a diagram that shows an element with its valence electrons shown as dots. They are very helpful in explaining the bonding properties of atoms and compounds. They can also determine the correct compound formula of a compound. However, they only work for elements in the s and p blocks (elements with less than 8 valence electrons).
Oxygen has 6 valence electrons. Once the s subshell is full with its electron pair, the p orbital gets the rest of the electrons. Hund’s rule says that every orbital in a subshell must be occupied by one electron before an electron pair can exist, so each of the 3 orbitals in the p subshell would have 1 electron, except for the first which would be paired. The Lewis dot structure for an oxygen atom would look like this:
One of the electron pairs comes from the s subshell, and the other 4 electrons are in the p subshell. Noble gases in the 18th family all have 8 valence electrons, which correlates to 4 electron pairs, or full s and p subshells.
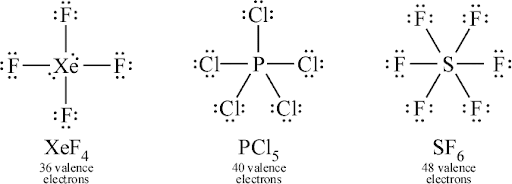
An advanced Lewis structure is any Lewis structure that has more than one element in it, creating a compound. There are a few rules for creating advanced Lewis structures.
Firstly, the amount of valence electrons should match the number of dots, unless it is a cation or anion, where you add or subtract an electron. Every atom should have the correct number of valence electrons, and the total should match the sum of all the valence electrons. The central atom should never be hydrogen, and it should be the element with the least electronegativity.
When bonding two atoms, a line is drawn connecting an electron on the first atom to an electron on the second atom. The atoms can also be double-bonded or even triple-bonded with other atoms, depending on the situation. When counting the valence electrons of a bonded element, you only count 1 for each bond that atom has, not both.
A single bond pairs two electrons from different atoms together. When the atom is double-bonded, it is in a covalent bond between two electron pairs, meaning the nuclei pull on both atoms equally. The more bonds there are, the stronger the bond is, and the closer together the atoms are.
Oxygen is a diatomic elementt, and when it is bonded with itself it has a structure like the one below.
The octet rule states that an atom has a tendency to have 8 valence electrons in a Lewis structure. When counting valence electrons for the octet rule, you count each bond as two electrons. This rule only applies to elements in the 3rd period or above.
Resonance is the idea of multiple possible Lewis structures. Delocalized bonding electrons don’t stay in one bond area, and they have the tendency to move around between areas. When there is a double or triple bond, they will often move around to other equally likely positions.
Carbonate, CO₃⁻², is a great example of this, as one of the oxygen atoms is double-bonded to the central carbon atom, whereas the other two are single-bonded to the carbon atoms. However, any of the three oxygen atoms being doubly bonded is equally possible, and therefore there are three resonance structures that need to be made for an accurate representation of the molecule.
Formal charge is a way of figuring out which possible Lewis structure is correct. It measures the exact charge of each element in a compound. A formal charge of 0 is favorable, but +/- 1 are also acceptable. To calculate formal charge, you count the number of valence electrons of an element, subtract the number of paired electrons, and then subtract half of the bonded electrons. For a Lewis structure to work, the sum of all formal charges must equal 0.
Carbon dioxide, CO₂ has three possible Lewis structures that obey the octet rule, but there is only one where all the formal charges are 0, which is more favorable than the other two. These are all possible CO₂ Lewis structures, where the first is the most favorable:
Since the world is in 3D, so are Lewis structures. This creates a theory known as VSEPR (valence shell electron pair repulsion). VSEPR basically states that the electron domains (electron locations) will repel each other with the maximum force, whilst also repelling other atoms.
This creates polar molecules because they are no longer always symmetrical. With a change in electron density, molecules can become polar. To determine if a structure is polar, you create a vector that corresponds with the dipole and then use vector addition to see if it is polar.
When looking at 3D molecular geometry (3D Lewis structures), there are six different classes based on how many electron domains and atoms are in a structure. Each class has at least 1 atom, and some electron domains which repel against each other and the atoms.
Class one molecules either have one atom and one electron domain (one electron or one pair) or two atoms. The shape of class one molecules will always be linear and polar unless there are two of the same atom (H-H bond), which will make it nonpolar.
Class two molecules are linear as well. They are only nonpolar when there are two electron domains attached to the central atom, or if there are two of the same atom attached to the central atom. There is also a 180ᵒ angle between the atoms/electrons, which accounts for the linear shape.
Class three molecules have a 120ᵒ angle between the atoms/domains, creating a shape called trigonal planar, which is a triangle that lies on one plane in 3D. They are always polar unless there are only electron pairs or the same atom. Note: for all classes except class one, the central atom does not matter for it to be nonpolar.
This pattern continues until class 6. However, since the electrons repel against everything else, the angles can change by a few degrees. For example: H₂O is a class four molecule, with two lone pairs and two attached atoms, which creates a bent shape. The predicted angle between atoms for this shape is 109.5ᵒ. However, the electrons repel them, creating an angle of around 104.5ᵒ.