Phase Changes
Thermodynamics is the relationship between heat, energy, temperature, and work. In simple terms, thermodynamics is the transfer of energy from one body to another. This change in energy is known as work in physics.
A system’s condition at any given time is known as its thermodynamic state. The state of a system is determined using the pressure, volume, and temperature of a gas.
Intermolecular forces, or IMFs, are forces that pull molecules together in matter. There are a few different types of IMFs, each with varying strength.
In chemistry, solids are defined as matter with a rigid, fixed shape and volume. Liquids are not rigid and have no fixed shape but still have a fixed volume. Gases are not rigid, have no fixed shape, and no fixed volume.
Solids have the strongest IMFs between their molecules, which is why it is much more rigid than the other two states of matter. The individual molecules are pulled together with relatively strong forces. Gases are barely pulled together and are therefore free to move around space.
Plasma is a superheated matter that is so hot that the electrons are ripped away, forming an ionized gas. In this case, it follows the same patterns as gaseous substances.
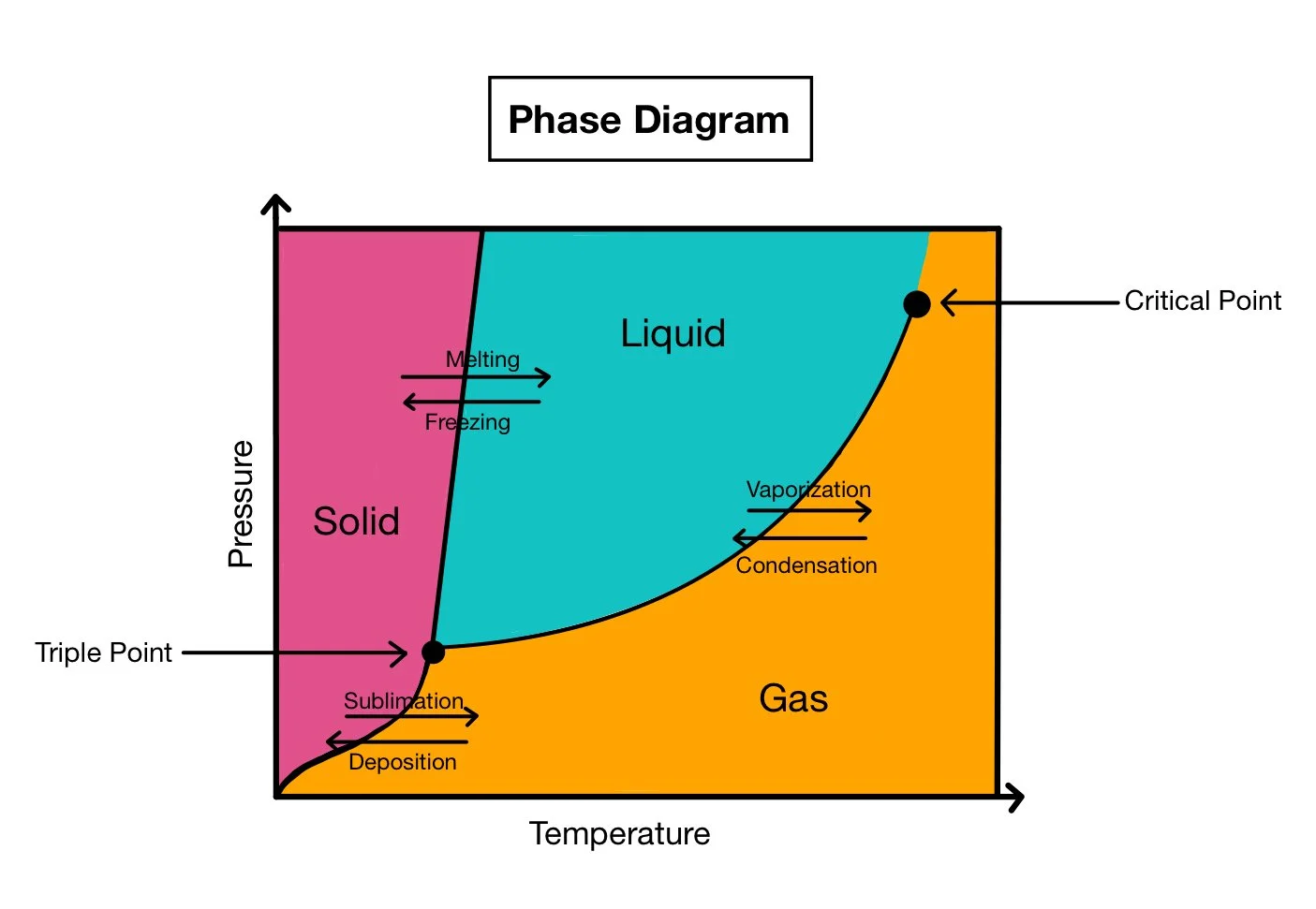
A phase change is when matter undergoes something which makes it change states of matter. The 8 phase changes are as follows: freezing, melting, vaporization, condensation, deposition, sublimation, ionization, and recombination. Due to changes in pressure or temperature, the molecules change their structure which causes a phase change. When the molecules are heated, they become more excited, causing them to move and separate.
Freezing is when a liquid phase transitions into a solid phase, and melting is the opposite, where a solid phase transitions to a liquid phase. This happens every year with many bodies of water.
Vaporization is when a liquid is transformed into a gas, such as water evaporating. Condensation is when the gas turns back into a liquid, which happens on many cups due to water vapor in the air.
Sublimation is when a solid turns straight into a gas without going through the liquid phase. Liquids cannot maintain their structure at certain temperatures and pressures, and solids turn straight to gas. The triple point is a temperature-pressure pair where all phases are the same. Dry ice is one substance with a high triple point relative to STP (1 atm and 0° C), which is why the solid CO₂ melts into a gaseous substance. Deposition is the reverse of sublimation.
Above the critical point, distinctions between liquid and gas do not exist. The substance is what is called a supercritical fluid, with properties of both gases and liquids. Examples of these are rare, like supercritical xenon, ethane, or carbon dioxide.
Ionization and recombination relate to plasma, where the gas loses its electrons at certain temperatures and ionizes into a plasma.